BLOOMINGTON, Ind.--(BUSINESS WIRE)--Cook Medical’s Women’s Health division has released the Guardia™ AccessET Curved Embryo Transfer Catheter, featuring Cook’s patented EchoTip® technology. The catheter’s echogenic band allows the catheter tip to be seen more clearly under ultrasound, which can allow for more accurate embryo placement.
Cook has also incorporated Microvol™ technology into the Guardia AccessET. Microvol decreases the volume of the fluid within the catheter that is required for embryo transfer. Less fluid can help reduce embryo migration and help optimize implantation.
The Guardia AccessET has a precurved guiding catheter that facilitates insertion into the uterus. Along with a bulb tip for easy passage through the cervix, the Guardia AccessET also has a soft, flexible transfer catheter that helps minimize endometrial trauma.
“The EchoTip technology used in the Guardia AccessET may significantly impact the ease of use for reproductive endocrinologists during a critical phase of the in vitro fertilization (IVF) process,” said Christina Anné, vice president of Cook Women’s Health business unit. “In vitro fertilization can be an emotionally and physically taxing experience for the patient. With that in mind, Cook continually seeks to provide fertility specialists with high-quality solutions that streamline and simplify the IVF process, while striving to improve overall outcomes and experiences for patients.”
Cook Medical and A.R.T.
Cook Medical is a global leader in assisted reproductive technology (A.R.T.). To aid A.R.T. professionals and their patients, Cook recently launched CookARTLab.com, featuring the industry's first interactive video tour of an A.R.T. lab. Visitors to the site can view real-world demonstrations of ovum collection, sperm injection, embryo culture and embryo transfer, with guidance on how to optimize each step for improved pregnancy rates. The website also features a knowledge base with up-to-the minute links to the most current published research in A.R.T.; a discussion forum that allows professionals to exchange information and experiences; and a news center that posts upcoming industry events—making the Cook site a centralized resource on breaking research and best practices for fertility and reproductive specialists.
About Cook Medical
Founded in 1963, Cook Medical pioneered many of the medical devices now commonly used to perform minimally invasive medical procedures throughout the body. Today, the company integrates medical devices, drugs and biologics to enhance patient safety and improve clinical outcomes. Since its inception, Cook has operated as a family-held private corporation. For more information, visit www.cookmedical.com. Follow Cook Medical on Twitter at twitter.com/cookmedicalpr and Cook Women’s Health at twitter.com/cookwomenshlth.
The Guardia™ AccessET is not available in Japan pending government approval.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6242398&lang=en
Contacts
Cook Urology and Women’s Health
Gail McDaniel, 812-339-2235 X7067
gail.mcdaniel@cookmedical.com
or
Racepoint Group, Inc.
Shannon Breen, 781-487-4607
sbreen@racepointgroup.com
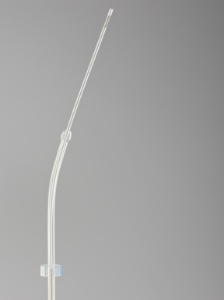
Guardia(TM) AccessET Curved Embryo Transfer Catheter features Cook Medical's patented EchoTip(R) technology that allows the catheter tip to be seen more clearly under ultrasound. (Photo: Business Wire)




