Postsurgical Incision Management Represents $1 Billion Global Market Opportunity: U.S. Approval Follows Prevena™ System Launch in Europe and Canada
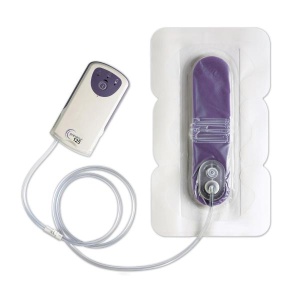
SAN ANTONIO--(BUSINESS WIRE)--Kinetic Concepts, Inc. (NYSE: KCI) announced today it has received 510(k) clearance from the Food and Drug Administration for the Prevena™ Incision Management System, a negative pressure wound therapy product designed for management of surgically closed incisions. KCI estimates more than three million procedures are performed each year worldwide that could benefit from treatment with the Prevena™ System, representing a potential global opportunity greater than $1 billion. The product will be available to hospitals in the U.S. in early July.
“KCI’s focus is on developing new technologies that improve patients’ lives, something we have done again with the launch of the Prevena™ System,” said Catherine Burzik, KCI’s president and CEO. “It’s another important milestone as we continue to execute on our growth strategy of commercializing a highly differentiated portfolio of products that help improve clinical outcomes and deliver tangible cost benefits.”
The Prevena™ System is indicated for use over clean, closed incisions that continue to drain following sutured or stapled closure. It acts by:
- Removing exudate and potentially infectious material
- Helping hold the edges of the incision together
- Protecting the surgical site from external contamination
- Providing a clean, protected postoperative wound environment
The Prevena™ System is designed for single-patient use. Portable and discreet, it accompanies the patient during transition from the hospital to the home.
“The Prevena™ Incision Management System represents the latest innovation to emerge from KCI’s negative pressure technology platform and strengthens our growing portfolio of products designed specifically for the surgical suite,” said Mike Genau, global president of KCI’s Active Healing Solutions™ business. “Postoperative incision complications are serious clinical challenges often associated with poor patient outcomes and increased hospital cost. The Prevena™ System is already getting high marks from surgeons in Europe and Canada who have told us it addresses an important unmet need in the surgical suite.”
Examples of complications that can follow surgical incisions include dehiscence, infection, hematoma and seroma. Sternal wound infections remain a serious complication from open-heart surgery, resulting in one-year mortality rates of up to 33 percent from mediastinitis after coronary artery bypass.1,2 The total annual cost to health care institutions for sternal wound infections alone has been estimated at more than $20 million.3
“Effective postoperative management of incisions is vital because complications such as infection and dehiscence can compromise patient quality of life, prolong hospital stays and increase overall cost of care,” said Mackenzie Quantz, MD, consultant, Cardiac Surgery, London Health Sciences Centre, London, Ontario and vice chair of the Specialty Committee in Cardiac Surgery at the Royal College of Physicians and Surgeons of Canada. “I have used the Prevena™ Incision Management System on a number of cardiac surgery patients who were at high risk for wound complications. The results have been promising.”
The Prevena™ System has been available in Canada and Europe since spring 2010.
About KCI
Kinetic Concepts, Inc. (NYSE:KCI), is a leading global medical technology company devoted to the discovery, development, manufacture and marketing of innovative, high-technology therapies and products for the wound care, tissue regeneration and therapeutic support system markets. Headquartered in San Antonio, Texas, KCI’s success spans more than three decades and can be traced to a history deeply rooted in innovation and a passion for significantly improving the healing and the lives of patients around the world. The company employs 6,800 people and markets its products in 20 countries. For more information about KCI, and how its products are changing the practice of medicine, visit www.KCI1.com.
Forward-Looking Statements
This press release contains forward-looking statements regarding management’s estimated market size for procedures eligible for use with the Prevena™ Incision Management System, as well as KCI’s expectations for product performance and plans for future product developments and introductions. Forward-looking statements may contain words such as believes, expects, anticipates, estimates, projects, intends, should, seeks, future, continue, or the negative of such terms, or other comparable terminology. Forward-looking statements are subject to risks, uncertainties, assumptions and other factors that are difficult to predict and that could cause actual results to vary materially from those expressed in or indicated by them. In particular, our ability to recognize increased sales and profitability from our Prevena™ Incision Management System products is subject to all the risks associated with the commercialization of new products based on innovative technologies, including unanticipated technical problems, manufacturing difficulties, gaining customer acceptance and other factors beyond our control. Additional risks and factors are identified in KCI’s filings with the U.S. Securities Exchange Commission (the SEC), including its Annual Report on Form 10-K for the fiscal year ending December 31, 2009, and Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, which are available on the SEC’s Web site at www.sec.gov. KCI undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise. All trademarks designated herein are proprietary to KCI Licensing, Inc. its affiliates and/or licensors.
1 Hollenbeak CS, Murphy DM, Koenig S, Woodward RS, Dunagan WC, Fraser VJ. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest. 2000;118:397-402.
2 Karra R, McDermott L, Connelly S, Smith P, Sexton DJ, Kaye KS. Risk factors for 1-year mortality after postoperative mediastinitis. J Thorac Cardiovasc Surg. 2006;132:537-543.
3 O’Reilly KB. Medicare’s no-pay events: coping with the complications. Amednews.com; July 14, 2008.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6329478&lang=en
Contacts
Kinetic Concepts, Inc.
Media:
Kevin Belgrade
Wireless: 210-216-1236
kevin.belgrade@kci1.com
or
Investors:
Todd Wyatt
Office: 210-255-6157
Wireless: 210-347-3540
todd.wyatt@kci1.com
PREVENA(TM) INCISION MANAGEMENT SYSTEM (Photo: Business Wire)




