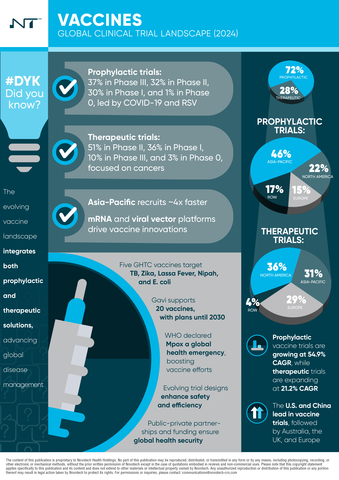
The whitepaper examines trends in prophylactic and therapeutic vaccine trials, highlighting advancements and emerging challenges. Key topics covered include the expansion of mRNA platforms, innovative delivery methods, and efforts toward equitable vaccine access by organizations such as WHO and Gavi.
Highlights:
- Emerging Trends: A shift towards adaptive trial designs, personalized vaccine trials, and novel delivery methods addressing infectious diseases and cancer.
- Regional Analysis: Clinical trial activity by region, emphasizing Asia-Pacific’s increasing role in vaccine development, especially in therapeutic vaccines.
- Investment Landscape: A summary of venture funding and M&A activity, with a focus on infectious and oncology vaccines.
- Regulatory Developments: Insights into streamlined regulatory processes established after COVID-19, aiming to expedite vaccine approvals and enhance pandemic preparedness.
The whitepaper is available for download, providing actionable data and strategic guidance on navigating regulatory complexities, patient recruitment, and leveraging new vaccine technologies.
For more details or to access the report, click here.
About Novotech Novotech-CRO.com
Founded in 1997, Novotech is a global full-service clinical Contract Research Organization (CRO) focused on partnering with biotech companies to accelerate the development of advanced and novel therapeutics at every phase.
Recognized for its industry-leading contributions, Novotech has received numerous prestigious awards, including the Frost & Sullivan 2024 Global Biotech CRO Award, 2024 Clinical Trials Arena Award for Excellence in Business Expansion, Marketing, and Innovation**,** 2024 Employer of Choice, 2024 Great Place to Work in the US, 2024 Brandon Hall Gold Award, CRO Leadership Award 2023, the Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023, the Asia-Pacific Contract Research Organization Company of the Year Award since 2006.
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical projects, including Phase I to Phase IV clinical trials and bioequivalence studies. With a presence in 34 office locations and a dedicated team of 3,000+ professionals worldwide, Novotech is a trusted end-to-end strategic partner of choice.
For more information or to speak to an expert team member visit www.Novotech-CRO.com